Moderna Applies For Emergency COVID Vaccine Approval In US & Europe Tyler Durden Mon, 11/30/2020 - 07:20
In a repeat of the last three Mondays, positive COVID vaccine news has delivered a jolt to stocks as Moderna has confirmed that it has applied for emergency approval. The company will ask regulators in the US (the FDA) and Europe, as the vaccine is set to become the second COVID-19 vaccine to go into service.
According to the company, in the 30,000-person trial, 196 subjects developed COVID-19 with symptoms after receiving either the vaccine or a placebo. Of those, 185 had taken a placebo, while only 11 had gotten the vaccine, indicating it protects against the disease.
If the FDA clears the shot, distribution could start within weeks. "I think this vaccine is going to be really a game changer for this pandemic," said Moderna Chief Executive Stéphane Bancel said in an interview with WSJ. "We think it can really prevent severe disease."
Minutes after the news broke, Bancel appeared on CNBC Monday morning to answer some questions. During the interview, he said the vaccine is "a premium product" intended for health-care workers, the elderly and others with "high risk". The company is in discussions with Covax, the international program to deliver vaccines to the developing world, to supply some of its vaccines to the program.
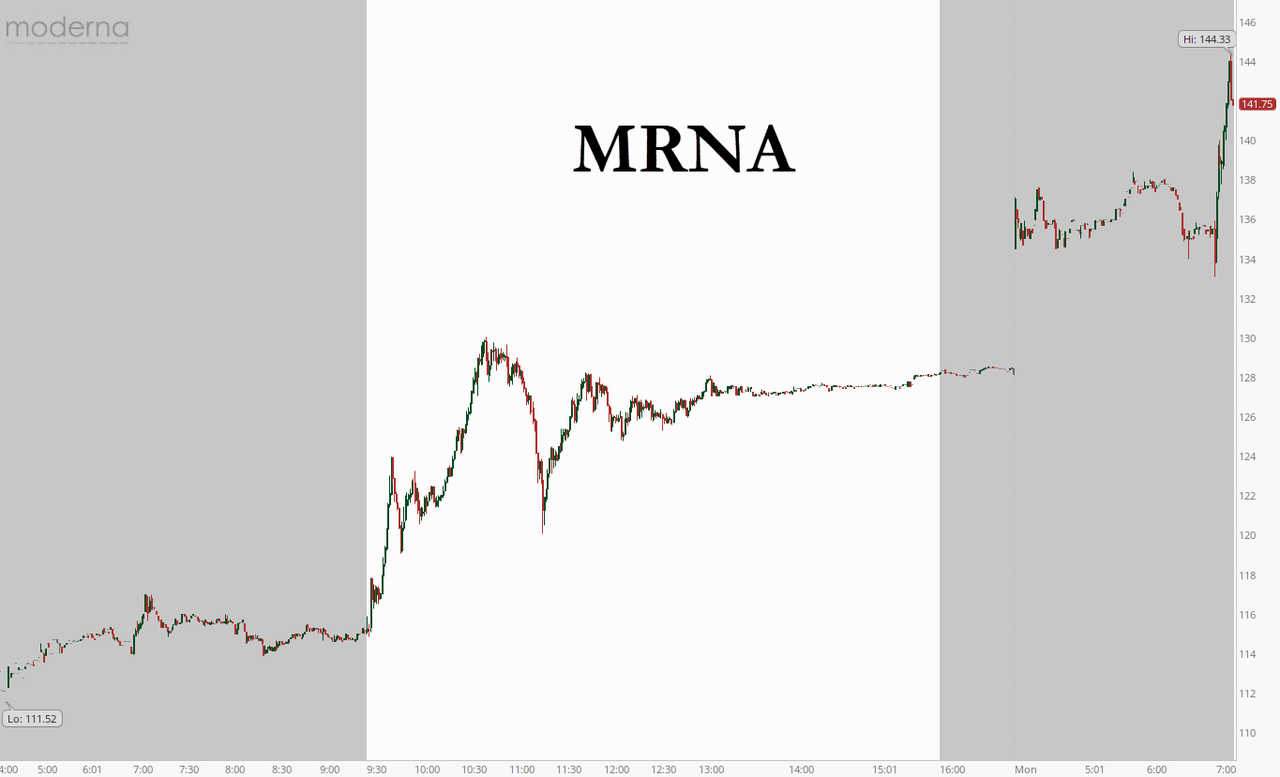
Moderna shares surged on the results, climbing more than 10% to their highest levels yet.
Bancel said that trials for minors - broken into two groups, teenagers and younger children - could start later this year and early next year.
Moderna two weeks ago reported preliminary results from the first 95 Covid-19 cases to emerge in the trial, indicating the shot was 94.5% effective and looking safe.
Since then, another 101 volunteers developed symptomatic disease, enough to finish the analysis and potentially satisfy regulators.
In the final analysis, the company said there were 30 severe cases among the 196 total cases of Covid-19, and that all of them were among people who had gotten the placebo.
The most common side effects, the company said, included pain around the injection site, fatigue and headache. Moderna said the reactions increase in frequency and severity after the second dose of the vaccine.
The company now has two months of safety data following the second dose for at least half of the study subjects—a threshold the FDA is requiring before authorizing any Covid-19 vaccine.
The 10-year old biotech company is based in Cambridge, Mass. Moderna expects to have 20 million doses by the end of the year, enough for about 10 million people. Pfizer also expects to have a limited supply of its vaccine this year.
https://ift.tt/2JcVWxf
from ZeroHedge News https://ift.tt/2JcVWxf
via IFTTT


0 comments
Post a Comment