"Grade-A Gaslighting": FDA Says Anti-Ivermectin Campaign Was 'Just A Recommendation'
The US Food and Drug Administration (FDA), which mocked Americans for taking Ivermectin to treat Covid-19, now says their campaign telling people to "stop" taking it was informal and just a recommendation, according to an argument made by government lawyers during a recent hearing.
"The cited statements were not directives. They were not mandatory. They were recommendations. They said what parties should do. They said, for example, why you should not take ivermectin to treat COVID-19. They did not say you may not do it, you must not do it. They did not say it’s prohibited or it’s unlawful. They also did not say that doctors may not prescribe ivermectin," Isaac Belfer, one of the lawyers, told the court during the Nov. 1 hearing in federal court in Texas according to the Epoch Times.
"They use informal language, that is true," Belfer continued, adding "it’s conversational but not mandatory."
Yet, in 2021 the FDA created a web page titled "Why You Should Not Use Ivermectin to Treat or Prevent COVID-19," before then tweeting: "You are not a horse. You are not a cow. Seriously, y’all. Stop it."
You are not a horse. You are not a cow. Seriously, y'all. Stop it. https://t.co/TWb75xYEY4
— U.S. FDA (@US_FDA) August 21, 2021
A second Twitter campaign stated: "Hold your horses, y’all. Ivermectin may be trending, but it still isn’t authorized or approved to treat COVID-19."
In a separate page noted by the Times, the FDA said: “Q: Should I take ivermectin to prevent or treat COVID-19? A: No.”
Jared Kelson, an attorney representing the plaintiffs, told the court during the hearing that that informal claim “doesn’t explain the language they actually used: ‘Stop it. Stop it with the ivermectin.'”
The FDA’s actions “clearly convey that this is not an acceptable way to treat these patients,” he argued.
Plaintiffs in the case include Dr. Paul Marik, who began utilizing ivermectin in his COVID-19 treatment protocol in 2020 while he was chief of pulmonary and critical care medicine at Eastern Virginia Medical School and director of the intensive care unit at Sentara Norfolk General Hospital.
After the FDA’s statements, Marik was told to remove the protocol from the school’s servers while Sentara issued a memorandum to hospitals telling them to stop using ivermectin against COVID-19, with a citation to the FDA. -Epoch Times
As Justin Heart, author of "Gone Viral," notes, the FDA is doing some "grade A gaslighting now."
Wow. Just wow. @US_FDA doing some grade A gaslighting now. “It was just a recommendation!” pic.twitter.com/qzKpjTpBIa
— Justin Hart (@justin_hart) November 20, 2022
This widely prescribed anti-parasitic which is also used in horses has shown massive efficacy worldwide in the treatment of mild and moderate cases of Covid-19, plus as a prophylactic. India's Uttar Pradesh province, with a population of over 200 million, says that widespread early use of Ivermectin 'helped keep positivity [and] deaths low.'
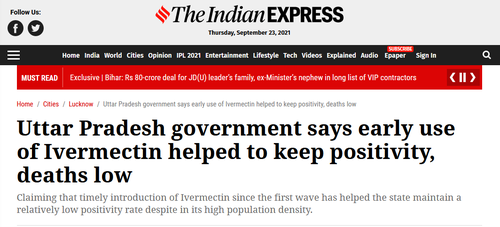
Separately, there have been several studies funded by the Indian government, primarily conducted through their largest govt. public medical university (AIIMS).
- Role of ivermectin in the prevention of SARS-CoV-2 infection among healthcare workers in India: A matched case-control study (source)
Conclusion: Two-dose ivermectin prophylaxis at a dose of 300 μg/kg with a gap of 72 hours was associated with a 73% reduction of SARS-CoV-2 infection among healthcare workers for the following month.
- Ivermectin as a potential treatment for mild to moderate COVID-19 – A double blind randomized placebo-controlled trial (source)
Conclusion: There was no difference in the primary outcome i.e. negative RT-PCR status on day 6 of admission with the use of ivermectin. However, a significantly higher proportion of patients were discharged alive from the hospital when they received ivermectin.
- Clinical Research Report Ivermectin in combination with doxycycline for treating COVID-19 symptoms: a randomized trial (source, double-blind randomized, peer-reviewed)
Discussion: In the present study, patients with mild or moderate COVID-19 infection treated with ivermectin in combination with doxycycline generally recovered 2 days earlier than those treated with placebo. The proportion of patients responding within 7 days of treatment was significantly higher in the treatment group than in the placebo group. The proportions of patients who remained symptomatic after 12 days of illness and who experienced disease progression were significantly lower in the treatment group than in the placebo group.
- Sharp Reductions in COVID-19 Case Fatalities and Excess Deaths in Peru in Close Time Conjunction, State-By-State, with Ivermectin Treatments (source, peer-reviewed, University of Toronto, Universidad EAFIT)
For the 24 states with early IVM treatment (and Lima), excess deaths dropped 59% (25%) at +30 days and 75% (25%) at +45 days after day of peak deaths. Case fatalities likewise dropped sharply in all states but Lima
- The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: A pilot, double-blind, placebo-controlled, randomized clinical trial (source, University of Barcelona, peer-reviewed)
Findings: Patients in the ivermectin group recovered earlier from hyposmia/anosmia (76 vs 158 patient-days; p < 0.001).
- A Comparative Study on Ivermectin-Doxycycline and Hydroxychloroquine-
Azithromycin Therapy on COVID-19 Patients (source - peer reviewed, though not govt funded)
Conclusion: According to our study, the Ivermectin-Doxycycline combination therapy has better symptomatic relief, shortened recovery duration, fewer adverse effects, and superior patient compliance compared to the Hydroxychloroquine-
Azithromycin combination. Based on this study's outcomes, the Ivermectin-Doxycycline combination is a superior choice for treating patients with mild to moderate COVID-19 disease.
- A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness (source, peer-reviewed double blind randomized, though small sample size)
Discussion: A 5-day course of ivermectin resulted in an earlier clearance of the virus compared to placebo (p = 0.005), thus indicating that early intervention with this agent may limit viral replication within the host. In the 5-day ivermectin group, there was a significant drop in CRP and LDH by day 7, which are indicators of disease severity.
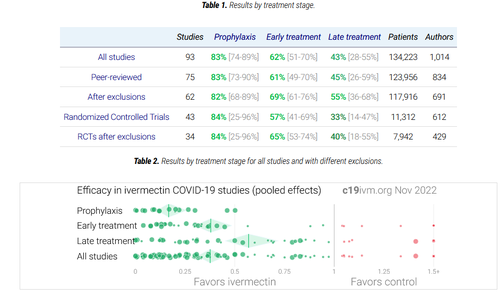
Meanwhile, There are currently 86 ongoing or completed clinical trials on Ivermectin around the world.
Drumroll...
https://ift.tt/yo9ubfL
from ZeroHedge News https://ift.tt/yo9ubfL
via IFTTT


0 comments
Post a Comment