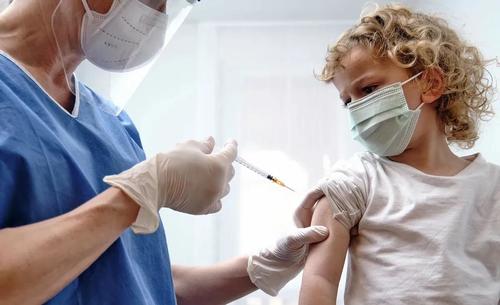
FDA Panel Votes In Favor Of Pfizer Covid-19 Vaccine For Children Aged 5 - 11
In a 17-0 vote, the US Food and Drug Administration (FDA) has recommended that the agency authorize the emergency use of Pfizer-BioNTech COVID-19 vaccine for children ages 5 through 11.
One member abstained from the vote.
The decision opens the door for final approval by the FDA, which could come as early as Wednesday, according to the Texas Tribune's Karen Brooks Harper.
The panel’s 17-0 vote, with one member abstaining, in favor of emergency use authorization for that age group opens the door for final approval by the FDA, which could come as early as Wednesday, by the FDA.
— Karen Brooks Harper (@kbrooksharper) October 26, 2021
As AP notes, the FDA isn't bound by the panel's recommendation.
Once approved, the Centers for Disease Control and Prevention (CDC) will render an opinion over children receiving the jab, and which groups should get them.
The dose for young children will contain one-third of the dose for those aged 12 and older.
Of note...
FDA Voting Member:
— Techno Fog (@Techno_Fog) October 26, 2021
"We're never gonna learn about how safe the vaccine is until we start giving it."
🤷♂️
Video HT @politicalwilli pic.twitter.com/OMAph49Qow
https://ift.tt/3BbWGaE
from ZeroHedge News https://ift.tt/3BbWGaE
via IFTTT

0 comments
Post a Comment